About Lasix® ONYU
Lasix ONYU is a novel drug-device combination product for the treatment of fluid overload in patients with heart failure.
Lasix ONYU was approved in October 2024 by the US Food and Drug Administration for the treatment of congestion due to fluid overload in adults with chronic heart failure.
Lasix is a rare enduring brand name that was first introduced 60 years ago. Lasix ONYU brings this iconic brand back in a state-of-the-art form — developed for the needs of our time.
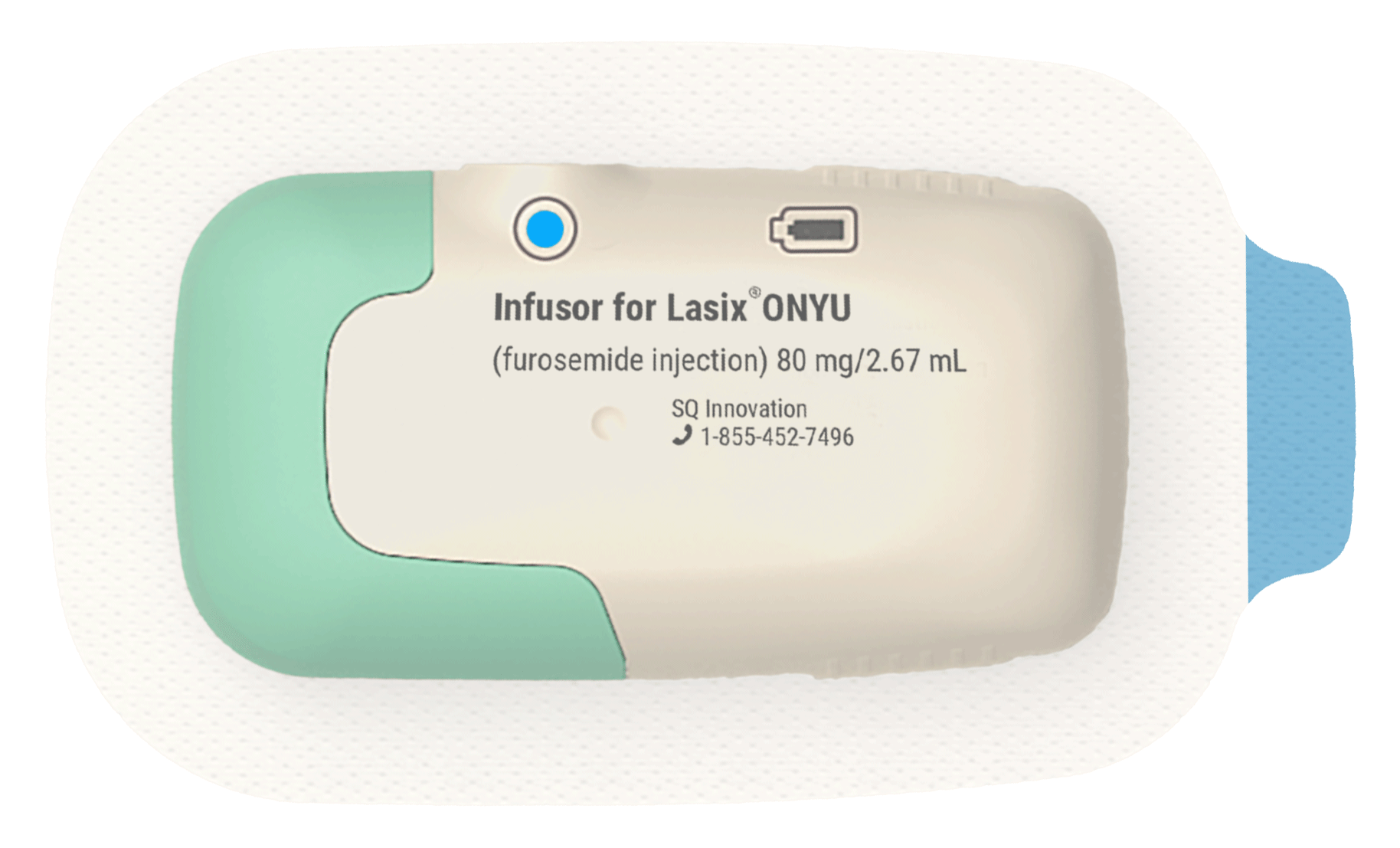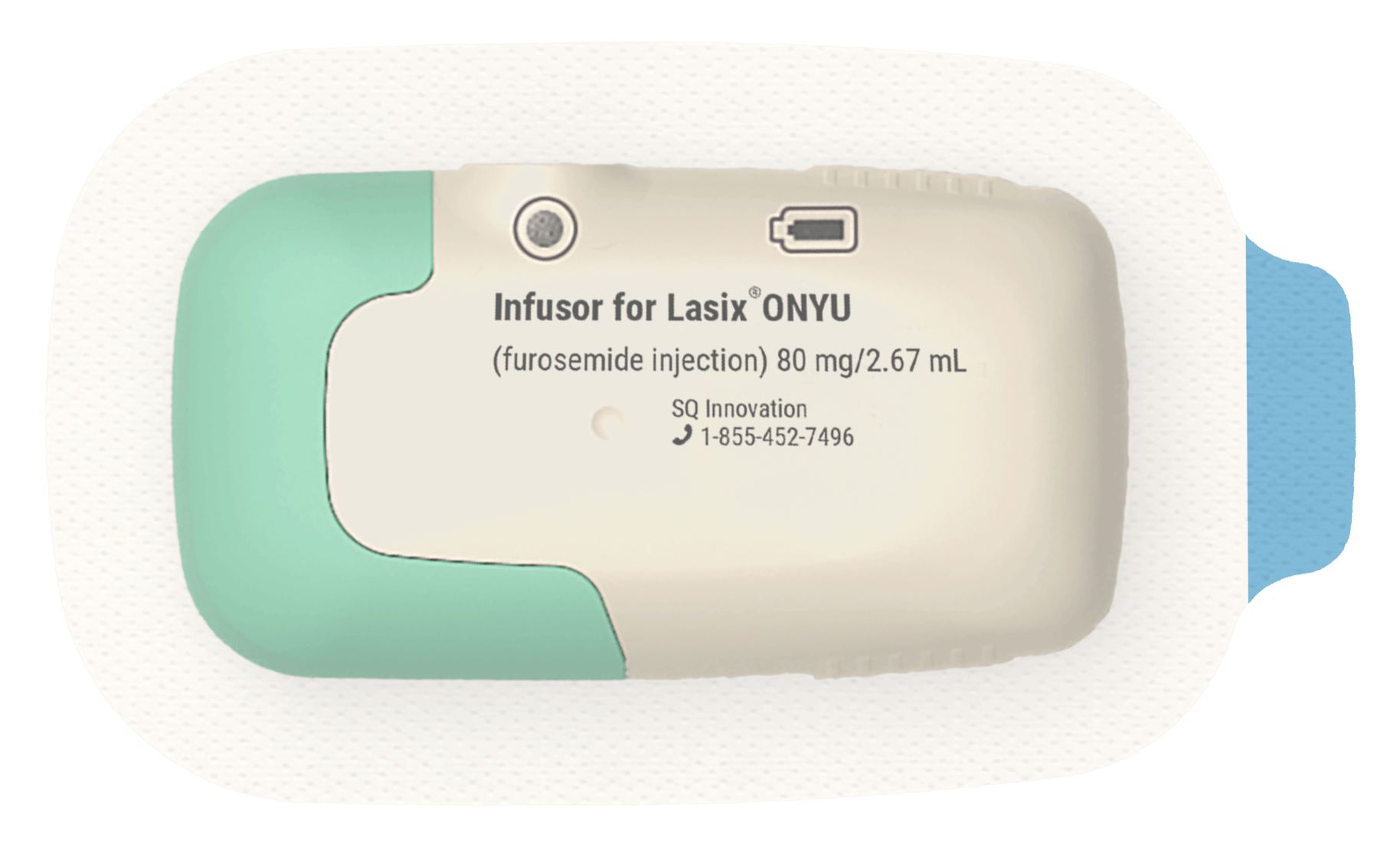
The Drug Component
Novel Furosemide Formulation
Formulated for Use with the Infusor
In order to fit in a small standard container we developed a novel formulation that contains 30mg of furosemide per mL — three times higher than the standard concentration of Furosemide Injection, USP. Just 2.67mL provides the therapeutic dose of 80mg. To reduce the risk of discomfort we made the pH about the same as the body (neutral pH). The formulation is made possible because of a special mixture of ingredients that we developed and patented.
Active Ingredient
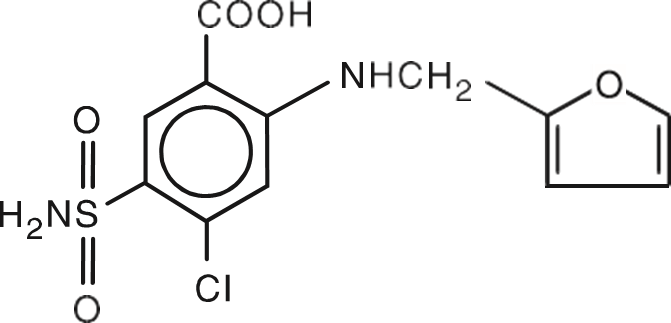
The active ingredient is the trusted Furosemide, USP.
Excipients
The excipient that made our proprietary formulation possible is betadex sulfobutyl ether sodium (300mg). Betadex is a well-know ingredient of pharmaceutical products that is made of starch. It is used in a variety of injectable products, eye and skin care products as well as dental products. In addition to betadex, it contains a small amount of tromethamine (3.0mg) solved in water for injection (q.s.). If needed, hydrochloric acid or sodium hydroxide may be added for pH adjustment to neutral.
pH
To reduce the risk of discomfort, the drug product is formulated to a pH of 7.5 (7.1 to 7.8) — lower than the standard furosemide injection, which typically has a pH around 9.0 (range 8.0 to 9.3).
The Device Component
The information below is for information only and not to be used to instruct a user. Users should read the Instructions for Use and follow the instructions of the healthcare provider.
The Infusor
A Two-Component Design
Our goal was to create a small pump that sticks to the abdomen to deliver furosemide subcutaneously in a controlled way over 5 hours. There were requirements that called for a sterile single-use disposable design while others called for use of electronics for actuation and advanced features. The solution we came up with was a two-component design with a sterile single-use disposable unit (DU) and a reusable unit (RU). The reusable unit can be used for 48 treatments. When a drug cartridge is inserted into the DU and paired with the RU, we refer to the complete device as the “Infusor”.
Disposable Unit
The DU is a sterile single-use component. It houses everything that is in contact with the drug solution and with the body. It comes in a sealed tray that should only be opened when it is ready to be used.

The DU contains a small pump that draws the drug solution from the cartridge and delivers it though a small needle under the skin. The needle is of the smallest kind used for injections. It is 29G which corresponds to about 1/3 of a mm or about half as thick as the needle used for vaccinations.
The needle comes out automatically after the Infusor is attached to the skin of the abdomen and the start button is pressed. When the treatment is finished it will pull itself back in the DU. If something goes wrong a red light indicates there maybe a risk of exposure to the needle and extra caution is required when removing and disposing the DU.
At the bottom of the DU is a white fabric medical tape to adhere to the skin of the abdomen. The small size and light weight allowed for use of a gentle tape (non-aggressive adhesive) to minimize discomfort and skin damage upon removal.
The DU is sterilized by gamma radiation. This the the preferred method for devices.
Reusable Unit
The RU contains small electromotor, batteries and electronics that control all its functions. It has two lights that inform the user of its status and operations. The motor of the RU rotates a pump shaft that connects with the pump in the DU.

The batteries of the RU are charged using a micro-USB wall charger that comes with the unit. It plugs in at the bottom of the RU before each use. It typically charges in about 5 minutes.
The RU informs the user of its status and operations. If an error is detected it stops and alerts the user. This is is explained in the Instructions for Use.
Designed for Lasix® ONYU
The Infusor was designed for the specific needs of the heart failure patient.
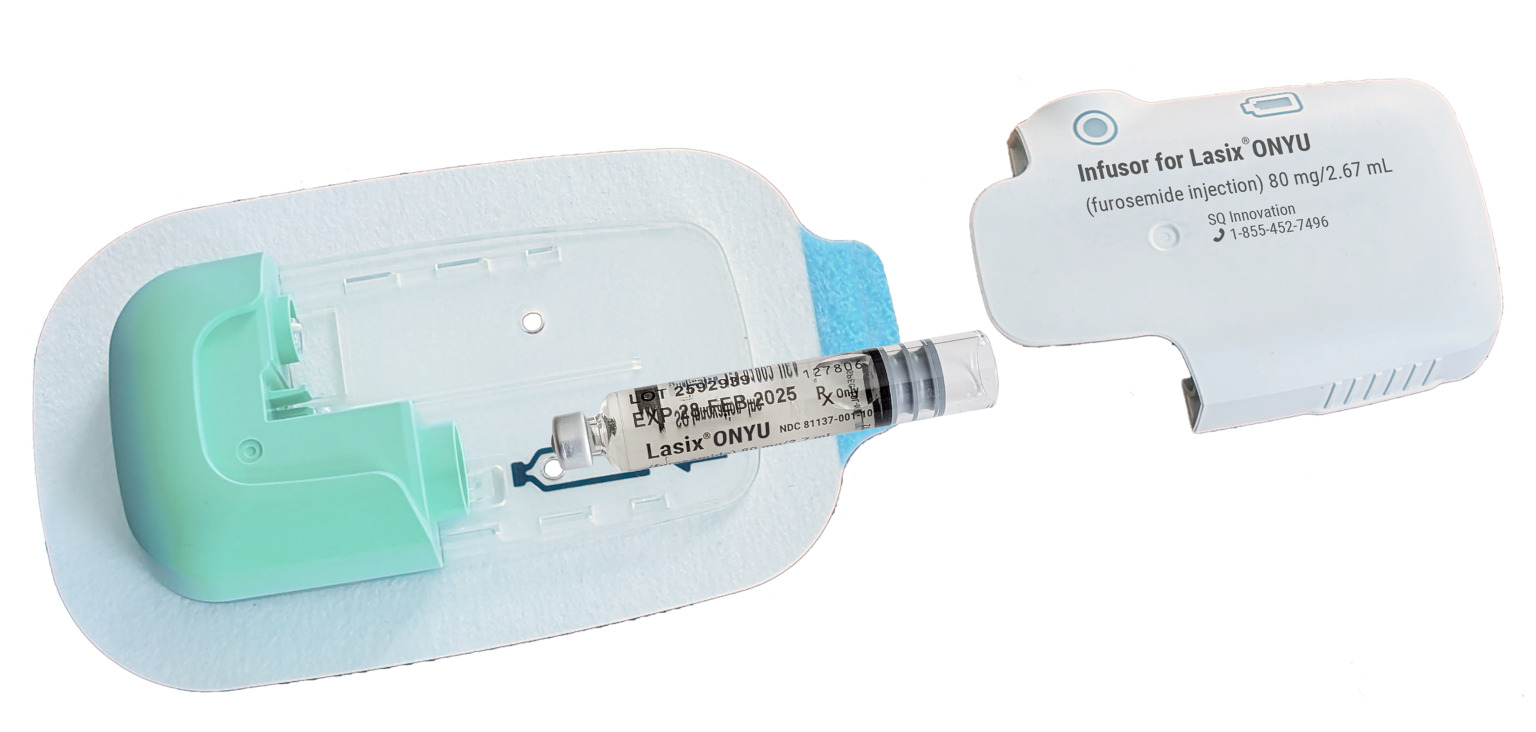
See Instructions for Use on how to prepare and operate the Infusor.
Lasix ONYU Treatment
The 5 Hour Treatment
See Prescribing Information for additional information.

Aiming for a Tempered but Full Response
The Objective
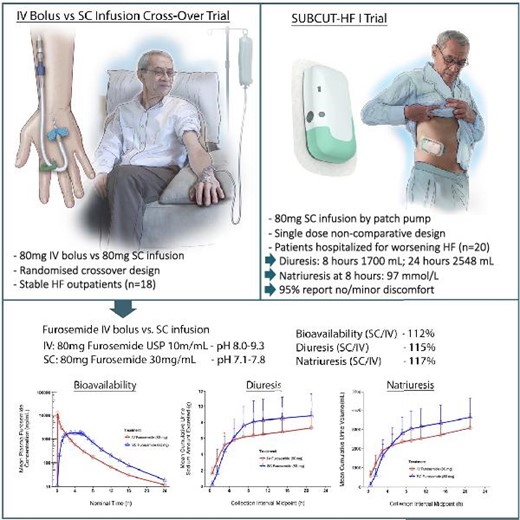
Furosemide has been used for over 50 years, mostly using oral tablets and intravenous administration by bolus (rapid injection over e.g., 2 minutes), or by IV drip. It is generally accepted that when you get certain amount of furosemide in circulation that you get a corresponding amount of diuresis, regardless if the drug is administered quickly (e.g., by IV bolus) or slowly (e.g., by drip). Although the amount of urine output is the same, the experience differs. In case of an IV bolus, there is a shorter, often very intense period of diuresis. With a slow infusion a similar amount of urine is produced over a longer period of time.
Considering patient preference and safety, we opted to deliver the 80mg dose over 5 hours, despite the Infusor’s capability for rapid administration. The safety related considerations included risk of incontinence, prostatism and orthostatic hypotension possibly resulting in falls and injury.
The Infusor delivers 30mg of furosemide over the first hour followed by 12.5mg per hour for 4 hours for a total of 80mg. With this a patient typically experiences marked diuresis over approximately 8 hours. In a clinical trial we conducted, the total volume of diuresis was similar to IV bolus, but more tempered over approximately 8 hours.
The 80mg Subcutaneous Infusion vs Individualising the IV Dose
Breaking the Mold
Furosemide Injection has been available since 1968 and at some point the concept emerged to select a dose of loop diuretics for IV treatment based on the daily home oral diuretic dose. However it is well established that bioavailability (percentage of drug that gets absorbed from the gut) varies from less than 20% to more than 90%. Consequently knowing the oral dose has no value for predicting what dose the patient may need. Diuretic response to loop diuretics is highly variable. A more pragmatic approach is to give a patient one or few standard doses of 80mg, then judge the response. Individualization is done by determining the number of total units needed to achieve the treatment goal. Up to two consecutive daily doses may be used, for example one at 8 am and the second one at 3 pm. In this case the patient will have very limited diuresis during the night to avoid interfering with sleep. In summary, the same important concept of empiric treatment remains — treat and observe — however it is now using a standard dose and individualize based on the number of treatments, not dose per treatment. For information about dosage and administration see the Prescribing Information.
Experience with Lasix® ONYU
In a study when the Infusor was compared to intravenous bolus of the same dose of 80mg, the Infusor produced complete bioavailability (112%). This means that all the furosemide delivered under the skin was found in the bloodstream. It is also called complete bioavailability. Details of the pharmacokinetic results of this study can be found in Section 12.3 of the Prescribing Information. In the same study, diuresis was very similar between the two treatments. After 8 hours, approximately 2.80L of urine was produced with Lasix ONYU compared to 2.35L with IV bolus. After 24 hours, it was 3.65L with Lasix ONYU and 3.09L with IV bolus.
The results of the study were published in a leading cardiovascular medical journal.
The Lasix ONYU Name — bringing a legend back
A Rare Enduring Brand*
The brand name Lasix® was first introduced in 1964 for prescription medicines containing furosemide. The name Lasix was used in the US and many countries around the world. In 1982 furosemide became generic in the US. Typically with passing time, the brand name makes place for use of the generic name. There are many prescription drugs still in widespread daily use where the current generation of prescribers do not know the original brand names. Somehow the Lasix name endured.
We consider our novel combination product worthy of the legendary brand name Lasix®. In order to make clear that this is not a standard form for injection we added “ONYU”. The technical term for name additions like this is “modifier”. The name Lasix® ONYU is fitting and should be easy to recognize and remember.
*The trademark LASIX® is registered for Validus Pharmaceuticals L.L.C. in the United States and used by SQ Innovation under license.


