SQ Innovation is a privately held Swiss biopharmaceutical company located in Zug, Switzerland, and Burlington, Massachusetts, United States.
Our company was established in 2019 to develop and commercialize innovative cost-effective therapies based on subcutaneous delivery of pharmaceutical products.
The parent company is located in Zug, Switzerland, but we operate out of our offices in Burlington, MA, just north of Boston.
Product innovation is our space. This is why we named the company SQ Innovation. We invented the now patented high concentration drug formulation. For our Infusor device, we partnered with Gerresheimer — a leading manufacturer of drug delivery systems and pharmaceutical drug containers headquartered in Germany.
At SQI we are committed to bringing innovative devices to the market that are of high-quality, affordable, and feature rich.
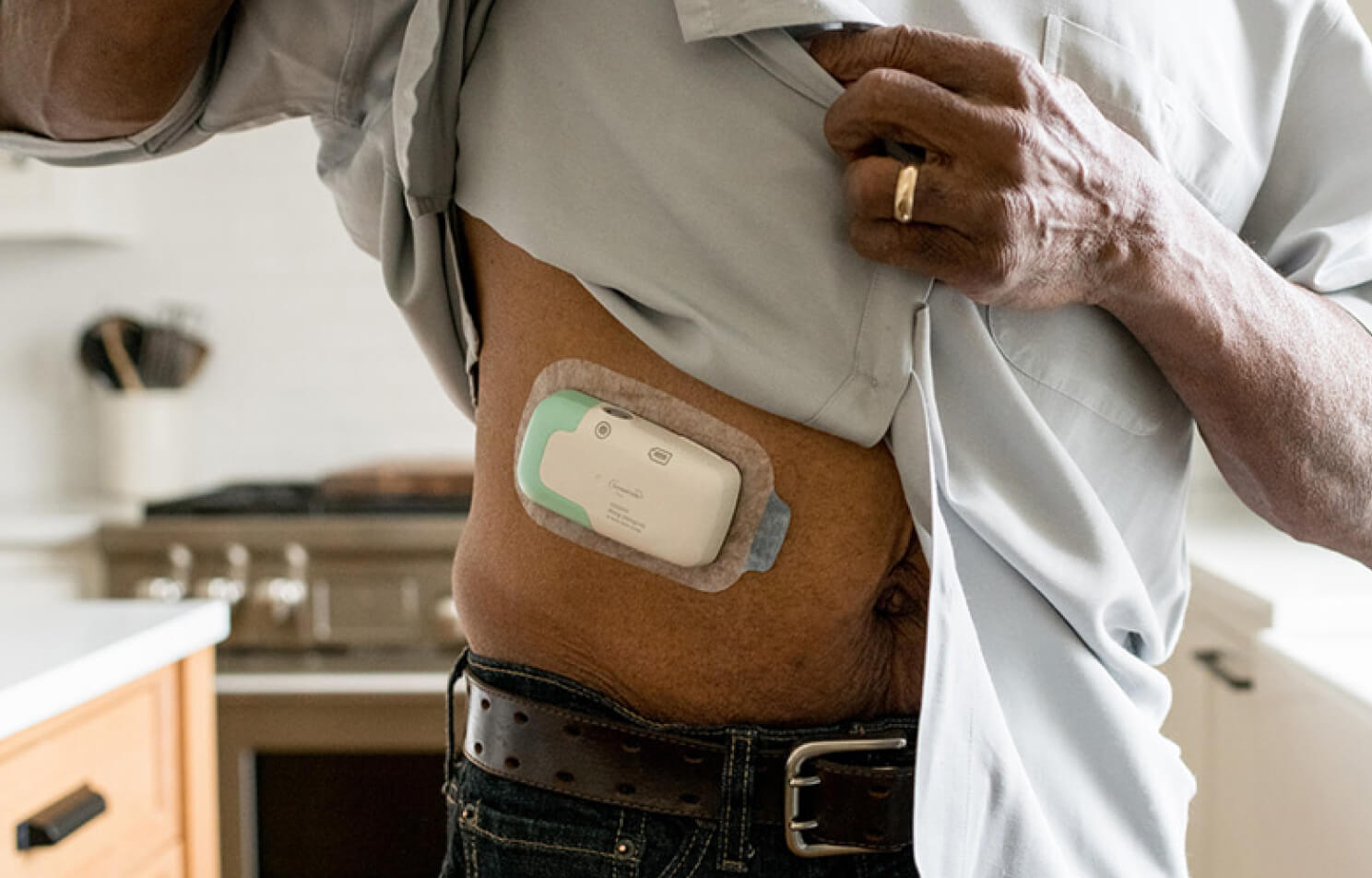
Our first product is a novel drug formulation of furosemide and an Infusor for the treatment of congestion due to fluid overload in patients with heart failure. It was approved by the US FDA in October of 2024 as Lasix ONYU. We are currently also pursuing regulatory approvals in Europe and the UK.
Contact Information
SQ Innovation, Inc
20 Mall Road, Suite 220
Burlington, MA 01803
USA
Telephone: +1 (781) 552-4990
Email: [email protected]
Our Values
Trust
We provide dependable products that meet customer expectations and improve patient care.
Talent
We value our people and their contributions towards operational excellence.
Innovation
We aim to design and develop transformative products for the benefit of patients and society.
Collaboration
We partner with our customers and suppliers so that we all win in the end,

